Synergistic effect of interfacial lattice Ag+ and Ag0 clusters in enhancing the photocatalytic performance of TiO2
Abstract
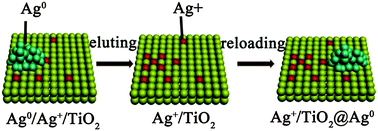
An interfacial lattice Ag+ doped on TiO2 (Ag+/TiO2) was prepared by eluting Ag0 clusters from a hydrothermally prepared Ag0/Ag+/TiO2 composite. An Ag+/TiO2@Ag0 composite photocatalyst was subsequently obtained via a secondary Ag0 clusters loading process to the Ag+/TiO2. The photocatalytic activity of Ag+/TiO2@Ag0 was greatly improved compared to Ag0/Ag+/TiO2 and Ag+/TiO2. X-ray photoelectron spectroscopy (XPS) and cyclic voltammetry (CV) testing verified that Ag+ ions occur as an interfacial lattice Ag+ species in the composites. The enhancement effect of the interfacial lattice Ag+ species is exhibited by the newly-formed Ag+/TiO2@Ag0 as the interfacial lattice Ag+ is fully exposed but not overlapped with the re-loaded Ag0 clusters. The interfacial lattice Ag+ ions and Ag0 clusters are both responsible for the photocatalytic performance improvement of the catalyst, in either the photocatalytic degradation of methyl orange or photocurrent measurement.

 Please wait while we load your content...
Please wait while we load your content...