Atomically dispersed rhodium on a support: the influence of a metal precursor and a support†
Abstract
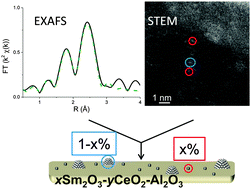
The influence of the support type and the metal precursor on the dispersion of rhodium after calcination and reduction was determined. The combination of electron microscopy and X-ray absorption analysis allowed the quantification of the amount of atomically dispersed rhodium in the samples. Higher amounts of atomically dispersed rhodium atoms are obtained when metal impregnation is performed with a rhodium acetate precursor in comparison to a rhodium chloride precursor over supports of the same composition. The stability of rhodium is improved with the addition of promoters; the co-presence of samaria and ceria in the support and metal impregnation with a rhodium acetate precursor leads to the highest amount of atomically dispersed rhodium remaining after reductive treatment at 773 K.
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...