An ab initio molecular dynamics study of thermal decomposition of 3,6-di(azido)-1,2,4,5-tetrazine†
Abstract
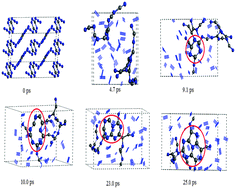
Ab initio molecular dynamics simulations were performed to study the thermal decomposition of isolated and crystal 3,6-di(azido)-1,2,4,5-tetrazine (DiAT). During unimolecular decomposition, the three different initiation mechanisms were observed to be N–N2 cleavage, ring opening, and isomerization, respectively. The preferential initial decomposition step is the homolysis of the N–N2 bond in the azido group. The release mechanisms of nitrogen gas are found to be very different in the early and later decomposition stages of crystal DiAT. In the early decomposition, DiAT decomposes very fast and drastically without forming any stable long-chains or heterocyclic clusters, and most of the nitrogen gases are released through rapid rupture of nitrogen–nitrogen and carbon–nitrogen bonds. But in the later decomposition stage, the release of nitrogen gas is inhibited due to low mobility, long distance from each other, and strong carbon–nitrogen bonds. To overcome the obstacles, the nitrogen gases are released through slow formation and disintegration of polycyclic networks. Our simulations suggest a new decomposition mechanism for the organic polyazido initial explosive at the atomistic level.

 Please wait while we load your content...
Please wait while we load your content...