Competing reactions of selected atmospheric gases on Fe3O4 nanoparticles surfaces†
Abstract
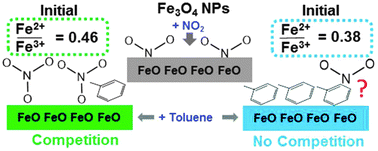
Heterogeneous reactions on atmospheric aerosol surfaces are increasingly considered important in understanding aerosol–cloud nucleation and climate change. To understand potential reactions in polluted atmospheres, the co-adsorption of NO2 and toluene to magnetite (Fe3O4i.e. FeO·Fe2O3) nanoparticles at ambient conditions was investigated for the first time. The surface area, size distribution, and morphology of Fe3O4 nanoparticles were characterized by BET method and high-resolution transmission electron microscopy. Adsorption isotherms, collected by gas chromatography with flame ionization detection, showed that the presence of NO2 decreased the adsorption of toluene. The analyses of the surface chemical composition of Fe3O4 by X-ray photoelectron spectroscopy (XPS) reveal that, upon the addition of NO2, the surface is oxidized and a contribution at 532.5 ± 0.4 eV in the O1s spectrum appears, showing that NO2 likely competes with toluene by dissociating on Fe2+ sites and forming NO3−. Different competing effects were observed for oxidized Fe3O4; oxidation occurred when exposed solely to NO2, whereas, the mixture of toluene and NO2 resulted in a reduction of the surface i.e. increased Fe2+/Fe3+. Analyses by time of flight secondary ion mass spectrometry further suggest toluene reacts with Fe3+ sites forming oxygenated organics. Our results indicate that on reduced magnetite, NO2 is more reactive and competes with toluene; in contrast, on oxidized Fe3O4, toluene is more reactive. Because magnetite can assume a range of oxidation ratios in the environment, different competing interactions between pollutants like NO2 and toluene could influence atmospheric processes, namely, the formation of Fe2+ and the formation of atmospheric oxidants.

 Please wait while we load your content...
Please wait while we load your content...