Nine-dimensional quantum dynamics study of the H2 + NH2 → H + NH3 reaction: a rigorous test of the sudden vector projection model
Abstract
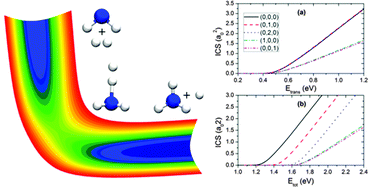
Reaction dynamics and mode specificity in the H2 + NH2 → H + NH3 reaction are investigated in full dimensionality on a recent ab initio based global potential energy surface. Integral cross sections from several low-lying vibrational states of both reagents have been calculated under the centrifugal sudden or J-shifting approximations, using an initial state selected time-dependent wave packet method. This nine-dimensional system provides an ideal proving ground to test our recently proposed Sudden Vector Projection (SVP) model. Our results indicate that vibrational excitation of H2 enhances the reactivity. On the other hand, excitation of either the symmetric or antisymmetric stretching mode of NH2 inhibits the reaction, while excitation of its bending mode has a negligible effect. Furthermore, all vibrational modes are less effective than translational energy in promoting the reaction. These mode-specific features are rationalized with the SVP model.

 Please wait while we load your content...
Please wait while we load your content...