The strong specific effect of coions on micellar growth from molecular-thermodynamic theory
Abstract
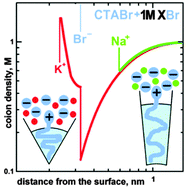
Viscoelastic solutions of ionic surfactants with an added salt exhibit a surprisingly strong dependence of their behavior on the nature of the added coion. We apply a recently proposed molecular-thermodynamic model to elucidate the effect of a coion's specificity on the aggregation of cationic and anionic surfactants. We show that micellar growth and branching are opposed by penetration of coions inside a micelle's corona leading to an increase of the aggregate's preferential curvature. These effects result from hydration/dehydration and dispersion attraction of coions and are only important at high salinity where electrostatic repulsion of coions from the micelle is screened and where branching of micelles and viscosity maxima are observed. At low and medium salinity, the coion plays a minor role; its effect on critical micelle concentration and sphere-to-rod transitions is insignificant. Our molecular-thermodynamic approach describes the specific effects of both counterions and coions and their different roles at different salinity levels based on a unified physical picture.

 Please wait while we load your content...
Please wait while we load your content...