Modelling potential/current distribution in microbial electrochemical systems shows how the optimal bioanode architecture depends on electrolyte conductivity
Abstract
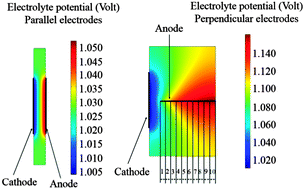
The theoretical bases for modelling the distribution of the electrostatic potential in microbial electrochemical systems are described. The secondary potential distribution (i.e. without mass transport limitation of the substrate) is shown to be sufficient to validly address microbial electrolysis cells (MECs). MECs are modelled with two different ionic conductivities of the solution (1 and 5.3 S m−1) and two bioanode kinetics (jmax = 5.8 or 34 A m−2). A conventional reactor configuration, with the anode and the cathode face to face, is compared with a configuration where the bioanode perpendicular to the cathode implements the electrochemical reaction on its two sides. The low solution conductivity is shown to have a crucial impact, which cancels out the advantages obtained by setting the bioanode perpendicular to the cathode. For the same reason, when the surface area of the anode is increased by multiplying the number of plates, care must be taken not to create too dense anode architecture. Actually, the advantages of increasing the surface area by multiplying the number of plates can be lost through worsening of the electrochemical conditions in the multi-layered anode, because of the increase of the electrostatic potential of the solution inside the anode structure. The model gives the first theoretical bases for scaling up MECs in a rather simple but rigorous way.

 Please wait while we load your content...
Please wait while we load your content...