Synthesis and spectroscopic properties of a soluble semiconducting porphyrin polymer
Abstract
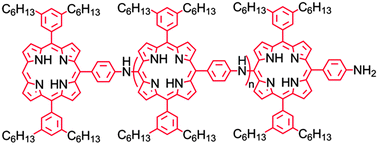
A semiconducting porphyrin polymer that is solution processable and soluble in organic solvents has been synthesized, and its spectroscopic and electrochemical properties have been investigated. The polymer consists of diarylporphyrin units that are linked at meso-positions by aminophenyl groups, thus making the porphyrin rings an integral part of the polymer backbone. Hexyl chains on two of the aryl groups impart solubility. The porphyrin units interact only weakly in the ground electronic state. Excitation produces a local excited state that rapidly evolves into a state with charge-transfer character (CT) involving the amino nitrogen and the porphyrin macrocycle. Singlet excitation energy is transferred between porphyrin units in the chain with a time constant of ca. 210 ps. The final CT state has a lifetime of several nanoseconds, and the first oxidation of the polymer occurs at ca. 0.58 V vs. SCE. These properties make the polymer a suitable potential excited state electron donor to a variety of fullerenes or other acceptor species, suggesting that the polymer may find use in organic photovoltaics, sensors, and similar applications.

 Please wait while we load your content...
Please wait while we load your content...