Stability of Pt near surface alloys under electrochemical conditions: a model study†
Abstract
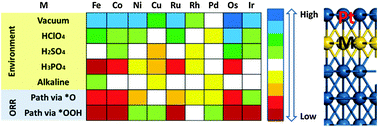
The stability is one of the key requirements for commercializing the fuel cell electrocatalysts in automotive applications. For the widely used Pt-based catalysts, it can be achieved by the formation of a stable Pt skin on the surface. Here, we employed density functional theory (DFT) to explore the stability of monolayer Pt (PtML) on various near surface alloy (NSAs) surfaces, PtML/MML/Pt(111) (M = Fe, Co, Ni, Cu; Ru, Rh, Pd, Ag; Os, Ir, Au), under various environmental conditions. Our results show that under the vacuum condition, the alloying M except Ag and Au thermodynamically prefer to stay in the subsurface and the formation of PtML on the surface is thermodynamically favored. A barrier has to be overcome for M to segregate. The situation varies under various electrochemical conditions. Depending on the solutions and the operating reaction pathway, different M should be considered for alloying with Pt to maintain the stability of surface PtML. PtRh and PtPd are the only two systems, where the surface PtML is likely to stay intact in perchloric acid (HClO4), sulfuric acid (H2SO4), phosphoric acid (H3PO4) and alkaline solutions as well as under the oxygen reduction reaction (ORR) conditions via different pathways. PtIr should also be paid attention, which falls only during the ORR via the OOH intermediate. Our results highlight the importance of chemical environments in affecting the stability of the catalysts.

 Please wait while we load your content...
Please wait while we load your content...