Mimicking anesthetic–receptor interactions in jets: the propofol–isopropanol cluster†
Abstract
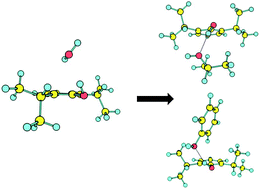
The interaction of the general anesthetic propofol with an individual residue of threonine in the membrane receptors has been modeled in the gas phase by examining the adduct of propofol with the isopropanol side-chain. We determined the structural preferences of the cluster using a combination of mass-resolved laser spectroscopy and quantum mechanical calculations. The first electronic transition of propofol–isopropanol was recorded with vibrational resolution using resonant two-photon ionization (R2PI) and ion dip IR spectroscopy. The spectra obtained were compared with density-functional calculations (DFT) using the M06-2X functional in order to obtain the cluster's structure. Three isomers have been detected. The results suggest that propofol acts as a Brønsted acid, donating a proton to the isopropanol molecule in a conformation that resembles that of propofol–water, but displaced towards the aromatic ring, due to the interaction with the aliphatic side of isopropanol. The higher affinity of propofol for isopropanol compared to water may correlate with the biological role of propofol at the protein binding site. On the other hand, propofol shows a similar affinity for isopropanol and phenol, which could explain the mobility that propofol experiences inside the GABAA cavity.

 Please wait while we load your content...
Please wait while we load your content...