On the infrared activation of the breathing mode of methane in ice
Abstract
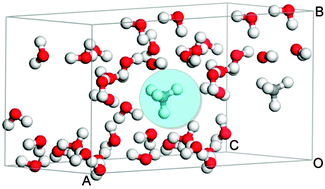
The symmetric stretching vibration (breathing mode) of methane is forbidden in the infrared spectra of gases. However, it has been observed in the spectra of low-pressure ice mixtures of methane and water, studied as models for astronomical ices. We investigate the possible origin of the activation of this mode by means of solid state calculations of amorphous water (ASW) samples into which methane molecules are introduced. Activation is predicted either by the interaction of the CH4 and H2O molecules in pore walls or via a strong mode coupling that takes place between the breathing mode of CH4 and the O–H stretching mode of H2O when both vibrations coincide in frequency. These two mechanisms would be favored for low-density or high density ASW, respectively. A possible experimental observation of this activation in compact ASW is discussed.

 Please wait while we load your content...
Please wait while we load your content...