Manipulating and probing enzymatic conformational fluctuations and enzyme–substrate interactions by single-molecule FRET-magnetic tweezers microscopy†
Abstract
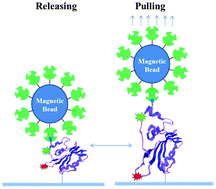
Enzyme–substrate interaction plays a critical role in enzymatic reactions, forming the active enzyme–substrate complex, the transition state ready to react. Studying the enzyme–substrate interaction will help in the ultimate molecular-level characterization of the enzymatic transition state that defines the reaction pathway, energetics, and the dynamics. In our initial effort to experimentally investigate the enzyme–substrate interactions and the related conformational fluctuations, we have developed a new approach to manipulate the enzymatic conformation and enzyme–substrate interaction at a single-molecule level by using a combined magnetic tweezers and simultaneous fluorescence resonance energy transfer (FRET) spectroscopic microscopy. By a repetitive pulling–releasing manipulation of a Cy3–Cy5 dye labeled 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK) molecule under the conditions with and without enzymatic substrates, we have probed and analyzed the enzymatic conformational dynamics. Our results indicate that the enzyme conformational flexibility can be regulated by enzyme–substrate interactions: (1) enzyme at its conformation-perturbed state has less flexibility when binding substrates, and (2) substrate binding to enzyme significantly changes the enzyme conformational flexibility, an experimental evidence of so called entropy trapping in the enzyme–substrate reactive transition state. Furthermore, our results provide a significant experimental analysis of the folding–binding enzyme–substrate interactions, a dynamic nature of the enzymatic active transition state formation process.

 Please wait while we load your content...
Please wait while we load your content...