Structural determination of Bi-doped magnetite multifunctional nanoparticles for contrast imaging†
Abstract
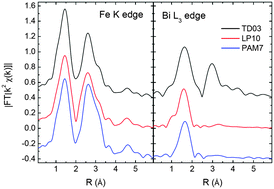
To determine with precision how Bi atoms are distributed in Bi-doped iron oxide nanoparticles their structural characterization has been carried out by X-ray absorption spectroscopy (XAS) recorded at the K edge of Fe and at the L3 edge of Bi. The inorganic nanoparticles are nominally hybrid structures integrating an iron oxide core and a bismuth oxide shell. Fe K-edge XAS indicates the formation of a structurally ordered, non-stoichiometric magnetite (Fe3−δO4) phase for all the nanoparticles. The XAS spectra show that, in the samples synthesized by precipitation in aqueous media and laser pyrolysis, the Bi atoms neither enter into the iron oxide spinel lattice nor form any other mixed Bi–Fe oxides. No modification of the local structure around the Fe atoms induced by the Bi atoms is observed at the Fe K edge. In addition, contrary to expectations, our results indicate that the Bi atoms do not form a well-defined Bi oxide structure. The XAS study at the Bi L3 edge indicates that the environment around Bi atoms is highly disordered and only a first oxygen coordination shell is observed. Indefinite [BiO6−x(OH)x] units (isolated or aggregated forming tiny amorphous clusters) bonded through hydroxyl bridges to the nanoparticle, rather than a well defined Bi2O3 shell, surround the nanoparticle. On the other hand, the XAS study indicates that, in the samples synthesized by thermal decomposition, the Bi atoms are embedded in a longer range ordered structure showing the first and second neighbors.

 Please wait while we load your content...
Please wait while we load your content...