Asymmetric criticality of binary ionic solutions containing 1-butyl-3-methylimidazolium tetrafluoroborate and alcohol†
Abstract
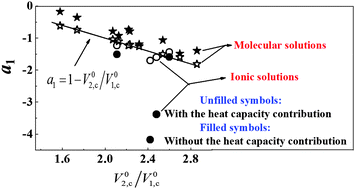
The liquid–liquid coexistence curves for binary solutions {1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim][BF4]) + 1-propanol} and {1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim][BF4]) + 2-propanol} have been precisely measured. The values of the critical exponents β obtained from the liquid–liquid equilibrium data in the critical region confirmed the 3D-Ising universality. The isobaric heat capacities per unit volume were measured for {[C4mim][BF4] + 1-propanol (or 2-propanol, 1,3-propanediol, 1,4-butanediol)} in both critical and non-critical regions. The experimental results indicate a major solvophobic contribution to the criticality for the studied ionic solutions. The complete scaling theory was applied to well represent the asymmetric behavior of the diameter of the coexistence curves with the consideration of the heat capacity contribution. It was found that the contribution of the heat capacity related term in the ionic solution decreased with the increase of the permittivity of alcohol and was more important in the description of the asymmetry of the coexistence curve of the ionic solutions than that of the molecular solutions.

 Please wait while we load your content...
Please wait while we load your content...