Direct measurements of the total rate constant of the reaction NCN + H and implications for the product branching ratio and the enthalpy of formation of NCN
Abstract
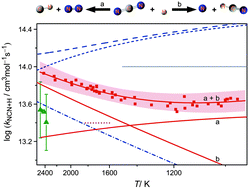
The overall rate constant of the reaction (2), NCN + H, which plays a key role in prompt-NO formation in flames, has been directly measured at temperatures 962 K < T < 2425 K behind shock waves. NCN radicals and H atoms were generated by the thermal decomposition of NCN3 and C2H5I, respectively. NCN concentration-time profiles were measured by sensitive narrow-line-width laser absorption at a wavelength of λ = 329.1302 nm. The obtained rate constants are best represented by the combination of two Arrhenius expressions, k2/(cm3 mol−1 s−1) = 3.49 × 1014 exp(−33.3 kJ mol−1/RT) + 1.07 × 1013 exp(+10.0 kJ mol−1/RT), with a small uncertainty of ±20% at T = 1600 K and ±30% at the upper and lower experimental temperature limits.The two Arrhenius terms basically can be attributed to the contributions of reaction channel (2a) yielding CH + N2 and channel (2b) yielding HCN + N as the products. A more refined analysis taking into account experimental and theoretical literature data provided a consistent rate constant set for k2a, its reverse reaction k1a (CH + N2 → NCN + H), k2b as well as a value for the controversial enthalpy of formation of NCN, ΔfHo298K = 450 kJ mol−1. The analysis verifies the expected strong temperature dependence of the branching fraction ϕ = k2b/k2 with reaction channel (2b) dominating at the experimental high-temperature limit. In contrast, reaction (2a) dominates at the low-temperature limit with a possible minor contribution of the HNCN forming recombination channel (2d) at T < 1150 K.

 Please wait while we load your content...
Please wait while we load your content...