CO2 activation and carbonate intermediates: an operando AP-XPS study of CO2 electrolysis reactions on solid oxide electrochemical cells†
Abstract
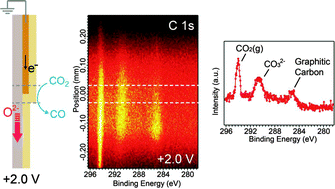
Through the use of ambient pressure X-ray photoelectron spectroscopy and specially designed ceria-based solid oxide electrochemical cells, carbon dioxide (CO2) electrolysis reactions (CO2 + 2e− → CO + O2−) and carbon monoxide (CO) electro-oxidation reactions (CO + O2− → CO2 + 2e−) over cerium oxide electrodes have been investigated in the presence of 0.5 Torr CO–CO2 gas mixtures at ∼600 °C. Carbonate species (CO32−) are identified on the ceria surface as reaction intermediates. When CO2 electrolysis is promoted on ceria electrodes at +2.0 V applied bias, we observe a higher concentration of CO32− over a 400 μm-wide active region on the ceria surface, accompanied by Ce3+/Ce4+ redox changes. This increase in the CO32− steady-state concentration suggests that the process of pre-coordination of CO2 to the ceria surface to form a CO32− intermediate (CO2(g) + O2−(surface) → CO32−(surface)) precedes a rate-limiting electron transfer process involving CO32− reduction to give CO and oxide ions (CO32−(surface) + 2Ce3+ → CO(g) + 2O2−(surface) + 2Ce4+). When the applied bias is switched to −1.5 V to promote CO electro-oxidation on ceria, the surface CO32− concentration slightly decreases from the equilibrium value, suggesting that the electron transfer process is also a rate-limiting process in the reverse direction.

 Please wait while we load your content...
Please wait while we load your content...