The effect of the ionic size of small quaternary ammonium BF4 salts on electrochemical double layer capacitors†
Abstract
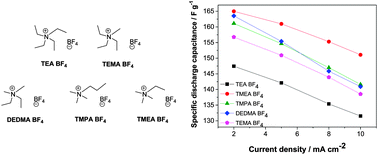
By varying the cation size of quaternary ammonium salts, approximately 10% higher capacitance was achieved with trimethylethylammonium BF4 and trimethylpropylammonium BF4 relative to tetraethylammonium BF4 using microporous activated carbon (AC) electrodes. The ions carried solvation shells in the bulk electrolytes, but became desolvated within the narrow AC pores when the electrochemical double-layer capacitor was charged to a high potential. The capacitance depended on the size of the cation rather than that of the BF4 anion because the anion is smaller than the quaternary ammonium ions. The capacitance was found to be proportional to the reciprocal radii of the neat cations. The effective radius of the asymmetric trimethylpropylammonium ion was estimated to be 0.314 nm based on the present results.

 Please wait while we load your content...
Please wait while we load your content...