On the possible biological relevance of HSNO isomers: a computational investigation†
Abstract
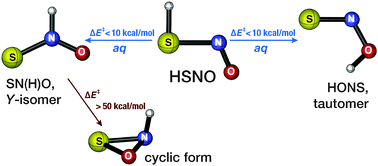
Thionitrous acid (HSNO), the smallest S-nitrosothiol, has been identified as a potential biologically active molecule that connects the biochemistries of two important gasotransmitters, nitric oxide (NO) and hydrogen sulfide (H2S). Here, we computationally explore possible isomerization reactions of HSNO that may occur under physiological conditions using high-level coupled-cluster as well as density functional theory and composite CBS-QB3 methodology calculations. Gas-phase calculations show that the formation of the tautomeric form HONS and the Y-isomer SN(H)O is thermodynamically feasible, as they are energetically close, within ∼6 kcal mol−1, to HSNO, while the recently proposed three-membered ring isomer is not thermodynamically or kinetically accessible. The gas-phase intramolecular proton-transfer reactions required for HSNO isomerization into HONS and SN(H)O are predicted to have prohibitively high reaction barriers, 30–50 kcal mol−1. However, the polar aqueous environment and water-assisted proton shuttle should decrease these barriers to ∼9 kcal mol−1, which makes these two isomers kinetically accessible under physiological conditions. Our calculations also support the possibility of an aqueous reaction between the Y-isomer SN(H)O and H2S leading to biologically active nitroxyl HNO. These results suggest that the formation of HSNO in biological milieu can lead to various derivative species with their own, possibly biologically relevant, activity.

 Please wait while we load your content...
Please wait while we load your content...