Significant enhancement of photocatalytic activity of rutile TiO2 compared with anatase TiO2 upon Pt nanoparticle deposition studied by far-ultraviolet spectroscopy†
Abstract
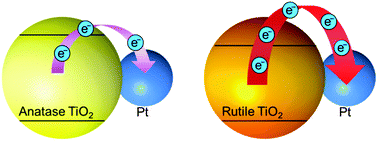
Absorption spectra of anatase and rutile TiO2 in the 150–300 nm region before and after the deposition of Pt nanoparticles were measured. For anatase TiO2, the spectral intensity in the longer wavelength region decreased (>∼210 nm), while that in the shorter wavelength region increased (<∼210 nm). In particular, spectral band intensity in the far-ultraviolet (FUV) region (∼160 nm) was increased. In contrast, the spectral intensity of rutile TiO2 increased over the entire wavelength region under investigation. Rutile TiO2 showed a spectral band at a longer wavelength region (∼170 nm) than anatase TiO2, and the difference in the band wavelengths in the FUV region was due to the differences in the electronic structures of their phase. The decrease and increase in the intensity upon the Pt nanoparticle deposition suggest electron transfer from the TiO2 to Pt nanoparticles and enhancement of charge-separation, respectively. The photocatalytic activity of rutile TiO2, as evaluated by a photo-degradation reaction of methylene blue, increased more than that of anatase TiO2 upon the deposition of Pt nanoparticles. Thus, we concluded that the charge-separation efficiency of rutile TiO2 is enhanced relative to that of anatase TiO2 upon the deposition of Pt nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...