CO2 incorporation in hydroxide and hydroperoxide containing water clusters—a unifying mechanism for hydrolysis and protolysis†
Abstract
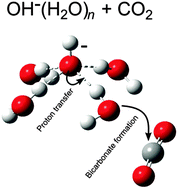
The reactions of CO2 with anionic water clusters containing hydroxide, OH−(H2O)n, and hydroperoxide, HO2−(H2O)n, have been studied in the isolated state using a mass spectrometric technique. The OH−(H2O)n clusters were found to react faster for n = 2,3, while for n >3 the HO2−(H2O)n clusters are more reactive. Insights from quantum chemical calculations revealed a common mechanism in which the decisive bicarbonate-forming step starts from a pre-reaction complex where OH− and CO2 are separated by one water molecule. Proton transfer from the water molecule to OH− then effectively moves the hydroxide ion motif next to the CO2 molecule. A new covalent bond is formed between CO2 and the emerging OH− in concert with the proton transfer. For larger clusters, successive proton transfers from H2O molecules to neighbouring OH− are required to effectively bring about the formation of the pre-reaction complex, upon which bicarbonate formation is accomplished according to the concerted mechanism. In this manner, a general mechanism is suggested, also applicable to bulk water and thereby to CO2 uptake in oceans. Furthermore, this mechanism avoids the intermediate H2CO3 by combining the CO2 hydrolysis step and the protolysis step into one. The general mechanistic picture is consistent with low enthalpy barriers and that the limiting factors are largely of entropic nature.

 Please wait while we load your content...
Please wait while we load your content...