Trapping RNase A on MCM41 pores: effects on structure stability, product inhibition and overall enzymatic activity†
Abstract
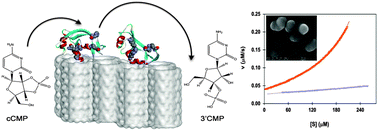
Catalytic activity of enzymes can be drastically modified by immobilization on surfaces of different materials. It is particularly effective when the dimensions of the biomolecules and adsorption sites on the material surfaces are commensurate. This can be utilized to hinder the biological activity of degradation enzymes and switch off undesired biological processes. Ribonucleases are particularly attractive targets for complete sequestration being efficient at disintegrating viable RNA molecules. Here we show that efficient quenching of ribonuclease A activity can be achieved by immobilization on the surface of MCM41 porous silica. Electron microscopy, isothermal titration calorimetry, differential scanning calorimetry and adsorption isotherm measurements of ribonuclease A on the MCM41 surface are used to demonstrate that the enzyme adsorbs on the external surface of the porous silica through electrostatic interactions that overcome the unfavorable entropy change as the protein gets trapped on the surface, and that immobilization shifts up its denaturation temperature by 20–25 °C. Real-time kinetic measurements, using single injection titration calorimetry, demonstrate that enzymatic activity towards hydrolysis of cyclic nucleotides is lowered by nearly two orders of magnitude on MCM41 and that active inhibition by the formed product is much less effective on the surface than in solution.

 Please wait while we load your content...
Please wait while we load your content...