Cyclic dipeptide immobilization on Au(111) and Cu(110) surfaces
Abstract
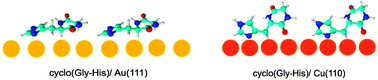
Soft X-ray Photoelectron Spectroscopy (XPS) and Near Edge X-ray Absorption Fine Structure (NEXAFS) spectroscopy have been used to probe the electronic and adsorption properties of two cyclic dipeptides, i.e. cyclo(glycyl-histidyl) and cyclo(phenylalanyl-prolyl), on Au(111) and Cu(110) surfaces. The core level spectra show chemical shifts which indicate weak chemisorption on Au(111), and stronger chemisorption on the Cu(110) surface, mainly via one of the nitrogen atoms in the central rings of both molecules, and nitrogen in the imidazole ring of cyclo(glycyl-histidyl). From the angular dependence of the NEXAFS spectra at the O and N K-edges, we conclude that both dipeptides have a preferred orientation on the two surfaces.

 Please wait while we load your content...
Please wait while we load your content...