Spin polarization gives rise to Cu precipitation in Fe-matrix
Abstract
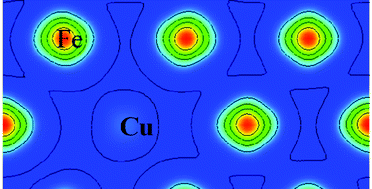
This article tries to uncover the physical reason of Cu precipitation from an Fe matrix at the electronic level. The general rule is obtained that the more bonds among Cu atoms, the more stable the system is. It was shown that Cu would precipitate from the matrix with Fe spin-polarization but not without spin-polarization. The partial density of states (PDOS) analysis illustrated that the d states of Fe near the Fermi level potentially have strong interaction with other atoms, but Cu d states below the Fermi level lack this potential, which results in weak covalent d orbital interaction between Fe and Cu. Furthermore, the charge density difference also confirmed the weaker bond between Fe and Cu with spin-polarization compared to without spin-polarization, due to the decreased charge between them. In addition, the {110} interface energy between Fe and Cu, estimated by the “dangling bond”, is 676.3 mJ m−2, which agrees with the DFT calculation, 414.2 mJ m−2. Finally, this study also revealed that Ni atoms can reduce the “dangling bond” when it locates at the interface and separates Fe and Cu.

 Please wait while we load your content...
Please wait while we load your content...