Photofragmentation spectroscopy of cold protonated aromatic amines in the gas phase†
Abstract
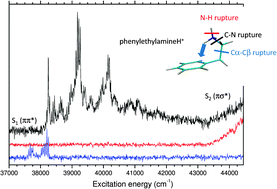
The electronic spectra of cold protonated aromatic amines: anilineH+ C6H5–NH3+, benzylamineH+ C6H5–CH2–NH3+ and phenylethylamineH+ C6H5–(CH2)2–NH3+ have been investigated experimentally in a large spectral domain and are compared to those of their hydroxyl homologues. In the low energy region, the electronic spectra are similar to their neutral analogues, which reveals the ππ* character of their first excited state. A second transition is observed from 0.4 to 1 eV above the origin band, which is assigned to the excitation of the πσ* state. In these protonated amine molecules, there is a competition between different fragmentation channels, some being specific to UV excitation i.e., not observed in low-energy collision induced dissociation experiments. Besides, for one amine a drastic change in the fragmentation branching ratio is observed within a very short energy range that reveals the complex excited state dynamics and fragmentation processes. The experimental observations can be rationalized using a simple qualitative model, the ππ*–πσ* model [A. L. Sobolewski, W. Domcke, C. Dedonder-Lardeux and C. Jouvet, Phys. Chem. Chem. Phys., 2002, 4, 1093–1100], which predicts that the excited state dynamics is controlled by the crossing between the ππ* excited state and a πσ* state repulsive along the XH (X being O or N) coordinate.

 Please wait while we load your content...
Please wait while we load your content...