Reactions of HOCO radicals through hydrogen-atom hopping utilizing clathrate hydrates as an observational matrix†
Abstract
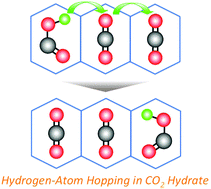
The carboxyl (HOCO) radical, which is an important species in atmospheric chemistry and combustion, is an intermediate in the reaction: CO + OH → CO2 + H and serves as a hydrogen donor to the reaction partners. The cis-HOCO radical, one of the ground-state HOCO radicals, is supposed to be decomposed into CO2 and the hydrogen atom by a tunneling effect. In order to prove the hypothesis, we performed electron spin resonance (ESR) measurements to investigate the decay mechanisms of the ground-state HOCO and DOCO radicals in gamma-ray-irradiated CO2 hydrates, which may hold the radicals stably. The ground-state HOCO and DOCO radicals decayed according to a second-order decay model and transformed into formic acid and CO2. The ratio of the decay rate constants of HOCO and DOCO radicals shows a good agreement with that in the kinetic isotope effect for the hydrogen and deuterium abstraction reactions. These results indicate that they react with another HOCO radical in the adjacent hydrate cage without the tunneling effect. This implies that the ground-state HOCO radicals are not decomposed by the tunneling effect but are decayed through reactions with some atoms, molecules, and/or radicals even in the gas phase. In addition, the hydrogen-atom hopping through the temporary hydrogen bonds between the HOCO radical and CO2 results in a seeming diffusion of the HOCO radicals in the CO2 hydrate; this would be an important concept for the studies of the radical diffusions and the supply of hydrogen atoms in gas, liquid, and solid phases.

 Please wait while we load your content...
Please wait while we load your content...