Energetics of disordered and ordered rare earth oxide-stabilized bismuth oxide ionic conductors
Abstract
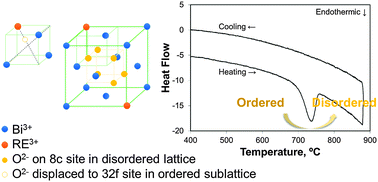
Rare-earth stabilized bismuth oxides are known for their excellent ionic conductivity at intermediate temperatures. However, previous studies have shown that their conductivity deteriorates during extended heat treatments at 500–600 °C, although the fluorite phase is maintained. In this study, the enthalpies of formation of quenched and aged ytterbia- and dysprosia-stabilized bismuth oxides were measured using high-temperature oxide melt solution calorimetry in 3Na2O–4MoO3 solvent at 702 °C. While a modest energy difference (−2 to −3 kJ mol−1) drives the kinetically slow aging transformation in the ytterbia-stabilized system at moderate dopant contents, no energetic driving force is detectable in the dysprosia-stabilized system. Although the small magnitude of the exothermic ordering energy suggests extensive short range ordering in both the quenched and aged samples, the anion configuration specific to the aged samples is nevertheless responsible for the significant decrease in conductivity.

 Please wait while we load your content...
Please wait while we load your content...