Mapping the structure of amyloid nucleation precursors by protein engineering kinetic analysis†
Abstract
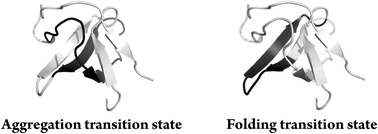
Understanding the early molecular mechanisms governing amyloid aggregation is crucial to learn how to prevent it. Here, we used a site-directed mutagenesis approach to explore the molecular mechanism of nucleation of amyloid structure in the N47A Spc-SH3 domain. The changes in the native state stability produced by a series of mutations on each structural element of the domain were uncorrelated with the changes in the aggregation rates, although the overall aggregation mechanism was not altered. Analysis of the thioflavin T initial rates based on a simple kinetic model allowed us to extract thermodynamic magnitudes of the precursor states of nucleation and map the regions of the protein participating in the structure of the amyloidogenic precursors. This structure differs from that of the folding transition state of the SH3 domains, strongly suggesting that the regions of the conformational landscape leading to amyloid formation are divergent from those leading to the native fold.

 Please wait while we load your content...
Please wait while we load your content...