Determination of volatility of ionic liquids at the nanoscale by means of ultra-fast scanning calorimetry†
Abstract
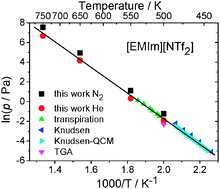
The determination of vaporization enthalpies of extremely low volatility ionic liquids is challenging and time consuming due to the low values of vapor pressure. In addition, these liquids tend to decompose even at temperatures where the vapor pressure is still low. Conventional methods for determination of vaporization enthalpies are thus limited to temperatures below the decomposition temperature. Here we present a new method for the determination of vaporization enthalpies of such liquids using differential fast scanning calorimetry. We have developed and proven this method using [EMIm][NTf2] at temperatures of up to 750 K and in different atmospheres. It was demonstrated that evaporation is still the dominating process of mass loss even at such highly elevated temperatures. In addition, since the method allows very high heating rates (up to 105 K s−1), much higher temperatures can be reached in the measurement of the mass loss rate as compared to common devices without significant decomposition of the ionic liquid. We discuss the advantages and limits of this new method of vaporization enthalpy determination and compare the results with data obtained from established methods.

 Please wait while we load your content...
Please wait while we load your content...