Shock wave and modeling study of the thermal decomposition reactions of pentafluoroethane and 2-H-heptafluoropropane
Abstract
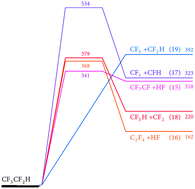
The thermal decomposition reactions of CF3CF2H and CF3CFHCF3 have been studied in shock waves by monitoring the appearance of CF2 radicals. Temperatures in the range 1400–2000 K and Ar bath gas concentrations in the range (2–10) × 10−5 mol cm−3 were employed. It is shown that the reactions are initiated by C–C bond fission and not by HF elimination. Differing conclusions in the literature about the primary decomposition products, such as deduced from experiments at very low pressures, are attributed to unimolecular falloff effects. By increasing the initial reactant concentrations in Ar from 60 to 1000 ppm, a retardation of CF2 formation was observed while the final CF2 yields remained close to two CF2 per C2F5H or three CF2 per C3F7H decomposed. This is explained by secondary bimolecular reactions which lead to comparably stable transient species like CF3H, releasing CF2 at a slower rate. Quantum-chemical calculations and kinetic modeling help to identify the reaction pathways and provide estimates of rate constants for a series of primary and secondary reactions in the decomposition mechanism.
- This article is part of the themed collection: PCCP’s 15th anniversary

 Please wait while we load your content...
Please wait while we load your content...