Structural analysis of neutral tetracycline using anharmonicity of delocalized vibrations
Abstract
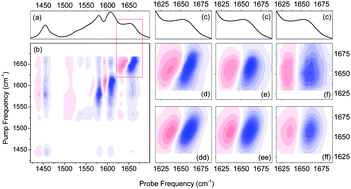
While tetracyclines are in active medical use, their bioactive atomic compositions are still questionable. Here, we investigate the structural properties of neutral tetracycline in dimethyl sulfoxide – the environment used often to mimic the environment in vivo. We compare the measured linear and nonlinear infrared spectra to those calculated for a collection of stable and energetically plausible tautomers, and describe the structurally sensitive off-diagonal peaks using anharmonicities of the normal modes. The comparison of experimental and theoretical 2DIR spectra is consistent with the numerical predictions of statistical thermodynamics on the relative weights of possible tautomers. In result, we provide the systematic account of the structural realizations of neutral tetracycline in DMSO.

 Please wait while we load your content...
Please wait while we load your content...