An ammonia-stabilized mixed-cation borohydride: synthesis, structure and thermal decomposition behavior†
Abstract
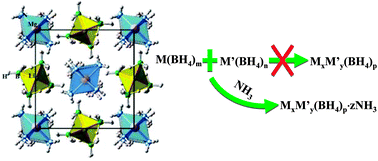
We demonstrate the synthesis, crystal structure and thermal decomposition behavior of a novel ammonia-stabilized mixed-cation borohydride where the NH3 groups enable the coexistence of Li and Mg cations as an “assistant”. Li2Mg(BH4)4·6NH3, which is comprised of orderly arranged Mg[NH3]62+ ammine complexes and Li2[BH4]42− complex anions, was synthesized by the mechanochemical reaction between Mg(BH4)2·6NH3 and LiBH4. This novel compound crystallizes in a tetragonal P43212 (No. 96) structure with lattice parameters a = b = 10.7656(8) Å and c = 13.843(1) Å with very short dihydrogen bonds, which determine a very low onset temperature of 80 °C for hydrogen release and are also responsible for the nucleation of Li2Mg(BH4)4·3NH3 as a decomposition intermediate. Mechanistic investigations on the thermal decomposition showed that the Hδ+–Hδ− combination in the ammonia-stabilized mixed-cation borohydride was significantly enhanced due to the strengthened Mg–N bonds. Upon heating, 11.02 moles of H2 (equivalent to 11.1 wt%) and 3.07 moles of NH3 are evolved from one mole of Li2Mg(BH4)4·6NH3 with a three-step reaction. The insights into the formation mechanism of ammonia-stabilized mixed-cation borohydride and the role played by NH3 group are very useful as a guideline for the design and synthesis of novel B–N-based materials with high hydrogen content.

 Please wait while we load your content...
Please wait while we load your content...