Is π halogen bonding or lone pair⋯π interaction formed between borazine and some halogenated compounds?†
Abstract
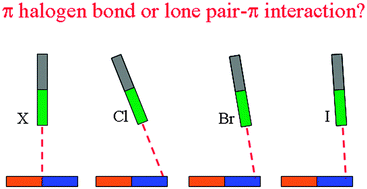
Borazine, “inorganic benzene”, exhibits some different properties from benzene although both of them are isostructural and isoelectronic. It was known that benzene is favorable to form halogen bonds with halogenated molecules. However, borazine more easily forms lone pair–π interactions with halogenated molecules, but for stronger halogen donors it can also form halogen bonds. The halogen bonds formed by borazine are stronger than the corresponding lone pair–π interactions. It was found that the pair–π interactions can be changed into halogen bonds with the increase of interaction strength. The dispersion energy plays a main role in stabilizing the weakly bonded complexes, while the electrostatic energy is dominant in the strongly bonded complexes. This is different from the nature of the respective benzene complexes.

 Please wait while we load your content...
Please wait while we load your content...