Vibronic spectra of the p-benzoquinone radical anion and cation: a matrix isolation and computational study†
Abstract
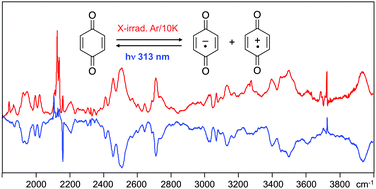
The electronic and vibrational absorption spectra of the radical anion and cation of p-benzoquinone (PBQ) in an Ar matrix between 500 and 40 000 cm−1 are presented and discussed in detail. Of particular interest is the radical cation, which shows very unusual spectroscopic features that can be understood in terms of vibronic coupling between the ground and a very low-lying excited state. The infrared spectrum of PBQ˙+ exhibits a very conspicuous and complicated pattern of features above 1900 cm−1 that is due to this electronic transition, and offers an unusually vivid demonstration of the effects of vibronic coupling in what would usually be a relatively simple region of the electromagnetic spectrum associated only with vibrational transitions. As expected, the intensities of most of the IR transitions leading to levels that couple the ground to the very low-lying first excited state of PBQ˙+ increase by large factors upon ionization, due to “intensity borrowing” from the D0 → D1 electronic transition. A notable exception is the antisymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, which contributes significantly to the vibronic coupling, but has nevertheless quite small intensity in the cation spectrum. This surprising feature is rationalized on the basis of a simple perturbation analysis.
O stretching vibration, which contributes significantly to the vibronic coupling, but has nevertheless quite small intensity in the cation spectrum. This surprising feature is rationalized on the basis of a simple perturbation analysis.

 Please wait while we load your content...
Please wait while we load your content...