Insights into the phosphatase and the synthase activities of human bisphosphoglycerate mutase: a quantum mechanics/molecular mechanics simulation
Abstract
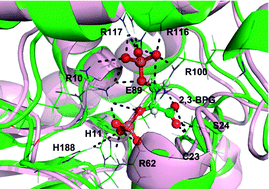
Bisphosphoglycerate mutase (BPGM) is a multi-activity enzyme. Its main function is to synthesize the 2,3-bisphosphoglycerate, the allosteric effector of hemoglobin. This enzyme can also catalyze the 2,3-bisphosphoglycerate to the 3-phosphoglycerate. In this study, the reaction mechanisms of both the phosphatase and the synthase activities of human bisphosphoglycerate mutase were theoretically calculated by using the quantum mechanics/molecular mechanics method based on the metadynamics and umbrella sampling simulations. The simulation results not only show the free energy curve of the phosphatase and the synthase reactions, but also reveal the important role of some residues in the active site. Additionally, the energy barriers of the two reactions indicate that the activity of the synthase in human bisphosphoglycerate mutase is much higher than that of the phosphatase. The estimated reaction barriers are consistent with the experimental data. Therefore, our work can give important information to understand the catalytic mechanism of the bisphosphoglycerate mutase family.

 Please wait while we load your content...
Please wait while we load your content...