From ultrafast events to equilibrium – uncovering the unusual dynamics of ESIPT reaction: the case of dually fluorescent diethyl-2,5-(dibenzoxazolyl)-hydroquinone†
Abstract
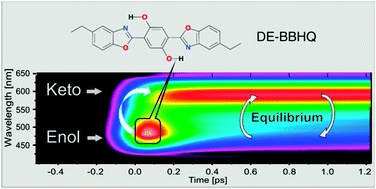
The excited state intramolecular proton transfer (ESIPT) reaction of the dually fluorescent 2,5-diethyl-(dibenzoxazolyl)-hydroquinone (DE-BBHQ) was studied with several time resolved techniques. The complementary character of up-conversion and time correlated single photon counting methods was demonstrated. According to the up-conversion experiments, the primary excited dienol form transforms into the monoketo tautomer in a very efficient ultrafast (∼100 fs) proton transfer reaction. The reverse process of proton transfer repopulating the excited dienol form was also observed, whose rate strongly depends on solvent polarity. Both contributions of dienol emission were univocally distinguished. The double-well potential of the S1 state of DE-BBHQ was calculated, and the nature of the phototautomer as the monoketo form was confirmed. This represents an example of how to combine different experimental methods with different temporal resolutions for unravelling ultrafast proton transfer reaction. A similar experimental strategy can be easily adopted for other systems where equilibrium between two states is observed (e.g. photoinduced electron or energy transfer).

 Please wait while we load your content...
Please wait while we load your content...