Substituent steering of dihedral angles around single bonds: the case of succinonitrile†
Abstract
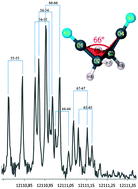
Succinonitrile is a material of plastic crystal nature arising from the low energy barrier between synclinal and antiperiplanar isomerization around the central C–C bond, while its high polarity makes it an efficient solvent for a wide variety of salts including ionic liquids. A prediction of the equilibrium dihedral angle – in the absence of experimental data – suffers from the shallow potential energy curve and electron diffraction results contain large standard errors. Here, to provide accurate structural data, the Fourier transform microwave spectrum and the mm-wave spectrum of the major isotopologues of synclinal succinonitrile have been measured, assigned and fitted to produce rotational, centrifugal distortion and quadrupole coupling constants. The mm-wave spectrum of the 13C and 15N singly substituted isotopologues in natural abundance has been assigned together with that of the chemically singly substituted 2H isotopologues. The resultant rotational constants have been used to calculate the substitution geometry for succinonitrile. All parameters and constants are compared with theoretical values computed at the B3LYP, MP2 and CCSD/cc-pVTZ levels of theory. The dihedral angle of succinonitrile, which is a strong driver of the plastic crystal nature of succinonitrile, is found to be 66(2)° best comparable to CCSD/cc-pVTZ predictions and noticeably different from the 60° expected without substituent effects.

 Please wait while we load your content...
Please wait while we load your content...