Modulation of glyceraldehyde-3-phosphate dehydrogenase activity by surface functionalized quantum dots†
Abstract
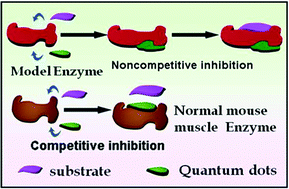
Enzymatic regulation is a fast and reliable diagnosis tool via identification and design of inhibitors for modulation of enzyme function. Previous reports on quantum dots (QDs)–enzyme interactions reveal a protein-surface recognition ability leading to promising applications in protein stabilization, protein delivery, bio-sensing and detection. However, the direct use of QDs to control enzyme inhibition has never been revealed to date. Here we show that a series of biocompatible surface-functionalized metal–chalcogenide QDs can be used as potent inhibitors for malignant cells through the modulation of enzyme activity, while normal cells remain unaffected. The in vitro activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an enzyme involved critically in the glycolysis of cancer cells, is inactivated selectively in a controlled way by the QDs at a significantly low concentration (nM). Cumulative kinetic studies delineate that the QDs undergo both reversible and irreversible inhibition mechanisms owing to the site-specific interactions, enabling control over the inhibition kinetics. These complementary loss-of-function probes may offer a novel route for rapid clinical diagnosis of malignant cells and biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...