The reactivity of CO2 on the MgO(100) surface
Abstract
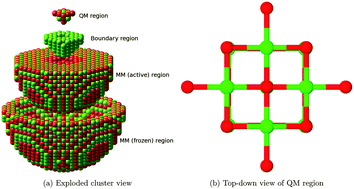
We investigate the adsorption of CO2 over an MgO(001) terrace, as calculated using an embedded cluster method. We find adsorbed geometries for CO2 on the perfect surface with energies which differ appreciably from previous studies, and observe that it is polarization of the surface rather than the inclusion of electron correlation which leads to this discrepancy. Our results suggest that both monodentate and tridentate carbonate formation on the MgO(001) surface are favourable processes, with the monodentate structure being of lower energy. Adsorption of CO2 is found to be favourable at both F0 and F+ terrace sites, but not at F2+. We also find that chemisorption at oxygen vacancy sites with a single localized electron (F+) could provide a route for the conversion of CO2 to other products, and that this system may be a useful model for other, more effective catalysts.

 Please wait while we load your content...
Please wait while we load your content...