The formation of carbamate ions in interstellar ice analogues†
Abstract
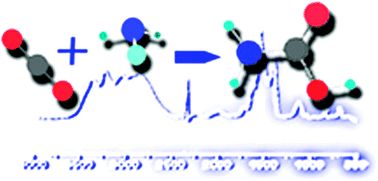
Carbon dioxide and ammonia are two of the most abundant species in astrophysical media, where they can react in the solid phase under certain conditions. This contribution presents a study of this reaction both in the presence of water and for anhydrous samples. It is shown that after deposition at 15 K, the reaction can start by warming the deposit, and the process continues on up to a temperature of 220 K. Reaction products are studied using infrared spectroscopy and mass spectrometry. For anhydrous samples, a 2 : 1 stoichiometry mixture of NH3 : CO2 gives the highest yield of products. The reaction is favored when a small amount of water is present, which enables ammonia and carbon dioxide molecules to collide within the pores and channels of the amorphous water solid. Large concentration of water, on the other hand, hampers such collisions. The main reaction product is found to be ammonium carbamate, but also carbamic acid is formed, and, in the presence of water, ammonium bicarbonate is produced as well. Theoretical calculations are carried out to provide the basis for the assignment of the spectra. Some of the experiments presented in this contribution consist of the generation of a compact water ice matrix where the carbamate and ammonium ions are embedded. If such a system was found in astrophysical media, it is shown that the ammonium ion could not be detected, whereas two infrared features of the carbamate ion in the 1040 to 1115 cm−1 (9 to 9.6 μm) region could enable the observation of this species.
- This article is part of the themed collection: Astrochemistry

 Please wait while we load your content...
Please wait while we load your content...