Oxygen release technique as a method for the determination of “δ–pO2–T” diagrams for MIEC oxides†
Abstract
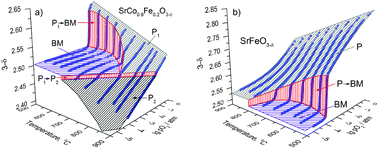
A new approach to the determination of oxygen nonstoichiometry (δ) of MIEC oxides as a continuous function of pO2 at high temperatures was developed. The description of the model allowing one to distinguish the contribution of oxygen released from the samples to the partial pressure of oxygen at the outlet of the continuous-flow fixed-bed reactor after the stepwise change of the oxygen partial pressure of inlet gas from 0.2 to 10−5 atm and to calculate the dependence of δ on pO2 is presented. The criterion for assessing the achievement of quasi equilibrium release of oxygen from the MIEC oxides is proposed. The adequacy of the method was confirmed by comparing the obtained and published data for well-studied SrCo0.8Fe0.2O3−δ and SrFeO3−δ MIEC oxides.

 Please wait while we load your content...
Please wait while we load your content...