Hydrogen bonding in microsolvation: photoelectron imaging and theoretical studies on Aux−–(H2O)n and Aux−–(CH3OH)n (x = 1, 2; n = 1, 2) complexes†
Abstract
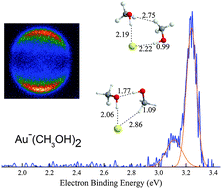
We have combined photoelectron velocity-map imaging (VMI) spectroscopy and theoretical calculations to elucidate the geometry and energy properties of Aux−(Solv)n clusters with x = 1, 2; n = 1, 2; and Solv = H2O and CH3OH. Besides the blue-shifted vertical electron detachment energies (VDEs) of the complexes Au1,2−(Solv)n with the increase of the solvation number (n), we independently probed two distinct Au−(CH3OH)2 isomers, which combined with MP2/aug-cc-pVTZ(pp) calculations represent a competition between O⋯H–O hydrogen bonds (HBs) and Au⋯H–O nonconventional hydrogen bonds (NHBs). Complementary calculations provide the total binding energies of the low-energy isomers. Moreover, the relationship between the total binding energies and total VDEshift is discussed. We found that the Au1,2− anions exhibit halide-analogous behavior in microsolvation. These findings also demonstrate that photoelectron velocity map imaging spectroscopy with the aid of the ab initio calculations is an effective tool for investigating weak-interaction complexes.

 Please wait while we load your content...
Please wait while we load your content...