Diversity in size of barium titanate nanocubes synthesized by a hydrothermal method using an aqueous Ti compound
Abstract
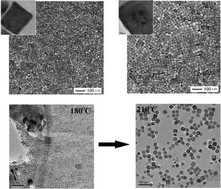
Barium titanate (BaTiO3) nanocubes with different size distributions were synthesized under hydrothermal conditions by using bis(ammonium lactate) titanium dihydroxide as the Ti source at a temperature of 220 °C. Oleic acid and tert-butylamine were employed as the surfactant and the additive in the synthesis of the nanocubes. tert-Butylamine was used to accelerate the formation of the BaTiO3 phase in the presence of oleic acid. The morphology and size of BaTiO3 nanocubes were controlled by tuning the barium and titanium precursor concentration and the molar ratio of the hydrothermal reaction system. The average size of the nanocubes was identified by dynamic light scattering which varied from ~15 nm to ~30 nm. High-resolution transmission electron microscopy and the fast Fourier transform (FFT) profile indicated that the BaTiO3 nanocubes were of single domain and possessed a cubic shape. Based on timely observation of the reaction conditions of the precursor solution during the hydrothermal process, it was found that a temperature of not less than 210 °C and a corresponding pressure higher than 2.2 MPa in this method played key roles in the synthesis of ~30 nm BaTiO3 nanocubes. Differential scanning calorimetry measurements were performed on BaTiO3 nanocubes and showed a broad exothermic transition around 70 °C.

 Please wait while we load your content...
Please wait while we load your content...