Influence of fluorine side-group substitution on the crystal structure formation of benzene-1,3,5-trisamides†
Abstract
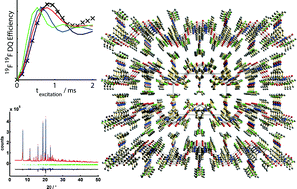
By a combination of powder X-ray diffraction, multidimensional and multinuclear solid-state NMR spectroscopy and quantum chemical calculations, we were able to determine the crystal structure of 1,3,5-tris(2-fluoro-2-methylpropionylamino)benzene. Solid-state NMR experiments guided the structure solution by predicting the content of the asymmetric unit and the presence of a NH⋯OC hydrogen bond network. In addition to real-space structure solution and Rietveld refinement, quantitative symmetry-based 19F19F double-quantum recoupling experiments provided a cost function to determine the positions of the methyl groups and fluorine atoms. The structure solution of this particular fluorine-substituted trisamide illustrates the impact of fluorine side-group substitution on the common columnar packing motif of benzene-1,3,5-tricarboxamides. As also in the case 1,3,5-tris(2,2-dimethylpropionylamino)benzene, the supramolecular aggregation is then guided by the formation of triple helical NH⋯OC hydrogen bond networks within the individual columns. In contrast, the substitution of one methyl group by a fluorine atom in each side chain results in a two-dimensional NH⋯OC hydrogen bond pattern, leading to a lamellar crystal structure with only van der Waals interactions between the layers. Since fluorine is not involved in the hydrogen bond network and both chemical units exhibit a similar steric demand, the fundamental differences of the packing are most probably caused by changes in the molecular polarity.

 Please wait while we load your content...
Please wait while we load your content...