Quasi-enantiomeric single-nucleoside and quasi-racemic two-nucleoside hydrochloride salts and ruthenium complexes of cytidine and 2′,3′-dideoxycytidine analogs unveiling the negligible structure-driving role of the 2′,3′-moieties†
Abstract
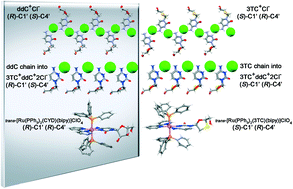
Insights into the structural basis of nucleoside molecular organization have been achieved from crystal engineering research. Here, we have investigated the role of differences at the 3′-position of the five-membered ring in the determination of the molecular conformation and crystal packing of quasi-enantiomeric cytidine-based nucleosides. Firstly, a hydrochloride salt of zalcitabine (2′,3′-dideoxycytidine) isostructural to lamivudine (2′,3′-dideoxy-3′-thiacytidine) hydrochloride was prepared. The R12(6) motif responsible for pairing between nucleosides and counterions as well as the assembly of chains and sheets with 21-screw axis symmetry-related ionic pairs were observed in both salts, even with lamivudine differing from zalcitabine in the opposite chirality of their C1′ and C4′ carbons and for a sulfur atom at the 3′-position rather than a methylene group. Based on the isostructurality between zalcitabine hydrochloride and lamivudine hydrochloride, but with crystal structures which are quasi-enantiomeric, a hydrochloride salt having both nucleosides in a single crystal form was designed in this study. The obtained hybrid structure is a quasi-racemate in which chains assembled with R12(6)-paired lamivudine and chloride can be related by pseudo inversion symmetry to that of zalcitabine and chloride but with an antiparallel growth direction. In addition, the first examples of ruthenium(II) coordination complexes with cytidine nucleosides are reported here. The trans-bis-(triphenylphosphine)(lamivudinate)(2,2′-bipyridine)ruthenium(II) perchlorate and trans-bis-(triphenylphosphine)(cytidinate)(2,2′-bipyridine)ruthenium(II) perchlorate complexes have the same coordination geometry and similar chains made up of complex units intercalated by water despite major molecular difference given by 2′-CH2 and 3′-S moieties in lamivudinate rather than CH2OH ones in both positions of cytidinate.

 Please wait while we load your content...
Please wait while we load your content...