Solid-state cis–trans isomerism in bis(oxamato)palladate(ii) complexes: synthesis, structural studies and catalytic activity†
Abstract
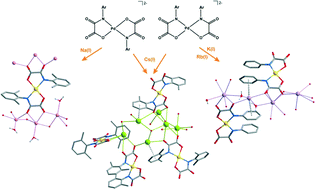
A new generation of bis(oxamato)palladate(II) monomeric complexes has been prepared by using N-2,6-dimethylphenyloxamate (2,6-Me2pma) as the ligand. Four alkaline salts of the complex, namely {[Na(H2O)]2trans-[PdII(2,6-Me2pma)2]}n (1a), {[Na4(H2O)2]cis-[PdII(2,6-Me2pma)2]2}n (1b), {[K4(H2O)3]cis-[PdII(2,6-Me2pma)2]2}n (2), {[Rb4(H2O)3]cis-[PdII(2,6-Me2pma)2]2}n (3) and {[Cs6(H2O)7]trans-[PdII(2,6-Me2pma)2]2cis-[PdII(2,6-Me2pma)2]}n·3nH2O (4), were obtained and structurally characterized by single crystal X-ray diffraction. Both the cis and trans stereoisomers of the [PdII(2,6-Me2pma)2]2− complex anion were isolated in the solid state, in a cation-dependent manner. The trans-isomer as the sodium salt (1a) crystallizes in the monoclinic space group I2/a with half of the molecule in the asymmetric unit. The cis isomer as sodium (1b), potassium (2) or rubidium (3) salt crystallizes in the acentric monoclinic space group Cc, with two whole molecules in the asymmetric unit. In the presence of cesium(I) (4), both the cis and trans stereoisomers co-crystallize in the orthorhombic system with Pbca as the space group, with two whole and two half molecules in the asymmetric unit. Previously reported square-planar bis(oxamato)metallate(II) [M = Cu(II) or Pd(II)] species with similar N-aryl substituted oxamate ligands only showed the occurrence of the trans isomer, complexes 1b and 2–4 offering unique examples of the alternative cis stereochemistry. The catalytic properties of 1a, 2–4 have been investigated in the palladium-catalyzed arylation of phenylboronic acid with a variety of aryl halides referred to as the Suzuki reaction, yielding biaryl compounds in very higher yields when iodobenzene [turnover number (TON) = 88–99 and turnover frequency (TOF) = 1056–1188 h−1] was used compared to commercial palladium(II) catalysts.

 Please wait while we load your content...
Please wait while we load your content...