Morphology-controllable Li2SiO3 nanostructures
Abstract
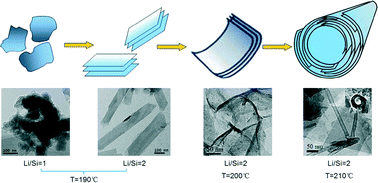
Hydrothermal synthesis of lithium metasilicate (Li2SiO3) has been systematically studied in aqueous alkaline environments by varying the Li/Si molar ratios of the solid materials and the hydrothermal temperatures. The phase structures and morphologies of the as-synthesized samples were investigated in detail by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy and N2 porosimetry. The morphology of Li2SiO3 varied from nanoparticles to nanosheets and nanotubes with the reaction conditions. A Li/Si molar ratio of 2 at 190 °C was ideal for the formation of pure nanosheets with a characteristic width of 100–200 nm and a typical length of 0.2–1.5 μm. Li2SiO3 nanoparticles were formed at lower Li/Si molar ratios, while most of the dispersed nanosheets curled to form nanotubes at higher Li/Si molar ratios. Nanotubes were formed at 210 °C and Li/Si molar ratio of 2, which possessed a typical inner diameter of ~20 nm, an outer diameter of 35 nm and a length ranging from 75 to 275 nm. An alkaline hydrothermal environment was beneficial to the formation of nanosheets and nanotubes. Atomic-level variations of the product structure from nanoparticles to nanosheets and nanotubes were depicted. A mechanism for the formation of different morphologies of Li2SiO3 was clarified.

 Please wait while we load your content...
Please wait while we load your content...