The role of secondary ammonium cations in controlling the conformation of C3-symmetric acid moieties and its implication for the design of supramolecular capsules†
Abstract
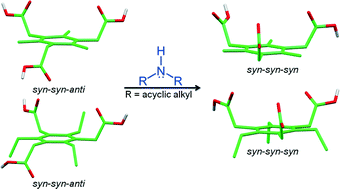
This article reports an attempt to synthesise salt based supramolecular capsules derived from two C3 symmetric tricarboxylic acids, namely 2,4,6-trimethylbenzene-1,3,5-triacetic acid (TMBTA) and 2,4,6-triethylbenzene-1,3,5-triacetic acid (TEBTA), having flexible carboxymethyl arms. Both acids displayed syn–syn–anti conformation of the carboxymethyl moiety as revealed by their single crystal structures. The secondary ammonium dicarboxylate (SAD) synthon concept was applied in order to bring all of the carboxymethyl arms into syn–syn–syn conformation conducive for capsular assembly. Reaction of various secondary amines with these two acids resulted in 16 salts, the majority of which turned out to be 1 : 1 (acid : amine) salts as revealed by FT-IR data. The single crystal structures of 10 such salts revealed that the desired capsules were not formed. However, in most of the structures, the carboxymethyl arms displayed syn–syn–syn conformation. The influence of the ammonium cations on the conformation of the flexible carboxymethyl arms of the acid moiety is discussed.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...