Effect of phenylacetic acid coligands on the structures and magnetic properties of azido-bridged copper(ii)-chain compounds†
Abstract
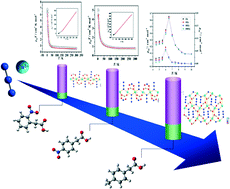
To explore and alter the structures of azido–copper(II) chain compounds, as well as investigate how their magnetic properties are influenced by substituents on the phenylacetic acid coligands, three new azido-bridged Cu(II) compounds, [Cu(o-npa)(N3)(H2O)]n (1), [Cu(p-npa)(N3)]n (2) and [Cu(p-mpa)(N3)]n (3) (o-Hnpa = o-nitrophenylacetic acid, p-Hnpa = p-nitrophenylacetic acid, and p-Hmpa = p-methylphenylacetic acid), have been successfully obtained, and structurally and magnetically characterized. Single-crystal structure analyses indicate that the azido ligands adopt the single end-on (EO) mode to connect adjacent Cu(II) centers in all three compounds. In compounds 1 and 2, the carboxylate groups exhibit the same μ2-bridging bidentate mode. Although compound 2 exhibits a 2D layer-structure formed through connections between the p-NO2 groups on the aromatic rings and 1D Cu(II)-chains, 2 shows similar intrachain ferromagnetic coupling to 1. When p-methylphenylacetic acid is employed as a coligand, compound 3 exhibits a 2D layer-structure, in which the carboxylate adopts a μ3-bridging tridentate mode to shorten the distance between Cu(II)-chains. Due to the counter-complementarity of the superexchange pathways in this system, compound 3 behaves as a magnet with a spontaneous magnetization temperature of 7 K.

 Please wait while we load your content...
Please wait while we load your content...