Hydrogen bonds in the crystal structure of hydrophobic and hydrophilic COOH-functionalized imidazolium ionic liquids†
Abstract
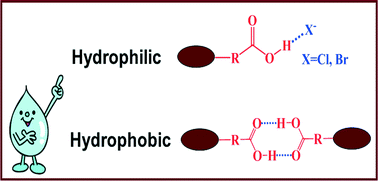
The crystal structures of six COOH-functionalized imidazolium ionic liquids (ILs) were determined, and different hydrogen bonds were found in the hydrophobic and hydrophilic ionic liquids. For the hydrophilic COOH-functionalized ILs based on halide anions, the classic O–H⋯Cl− or O–H⋯Br− hydrogen bond was observed by single crystal X-ray diffraction, whereas the carboxyl group dimer was present for the hydrophobic COOH-functionalized ILs based on the (CF3SO2)2N− (TFSI) and PF6− anions. Parallel vibrational spectroscopic studies and DFT calculations have also demonstrated the difference in the hydrogen bond structures. The relationship between the solubility of the ionic liquids in water and their structures was discussed.

 Please wait while we load your content...
Please wait while we load your content...