Dehydration of mildronate dihydrate: a study of structural transformations and kinetics†
Abstract
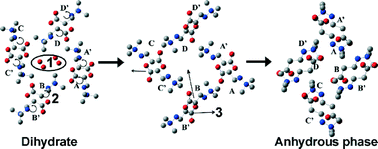
The dehydration of mildronate dihydrate (3-(1,1,1-trimethylhydrazin-1-ium-2-yl)propionate dihydrate) was investigated by powder X-ray diffraction, thermal analysis, hot-stage microscopy, water sorption–desorption studies and dehydration kinetic studies. It was determined that mildronate dihydrate dehydrated in a single step, directly transforming into the anhydrous form. In order to understand the reasons for a one step dehydration mechanism, crystal structures of dihydrate, monohydrate and anhydrous forms were compared, proving the similarity of the dihydrate and anhydrous forms. In order to understand the reasons for molecule reorganization during dehydration, the energy of the anhydrous form was compared with that of a theoretical dihydrate structure without water molecules. It was proven that the experimentally observed anhydrous phase AP was thermodynamically more stable. By analyzing the effect of the particle size and sample weight on the dehydration kinetic parameters it was determined that besides the main rate limiting step, phase boundary advancement, contribution from the water diffusion outside the crystal and the water diffusion outside the powdered sample also appeared to affect the dehydration kinetics and contribution from these processes could be changed by changing the aforementioned factors.

 Please wait while we load your content...
Please wait while we load your content...